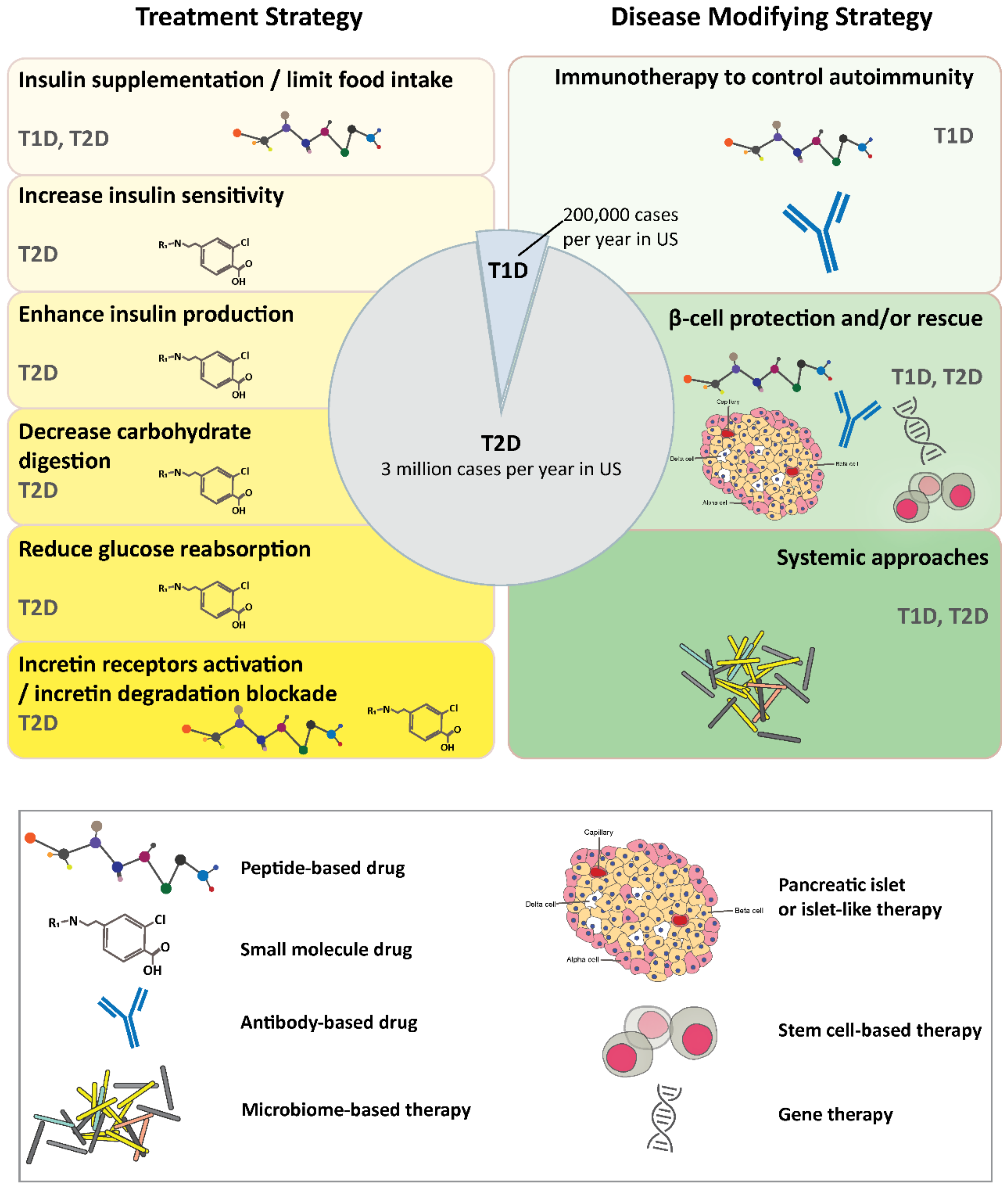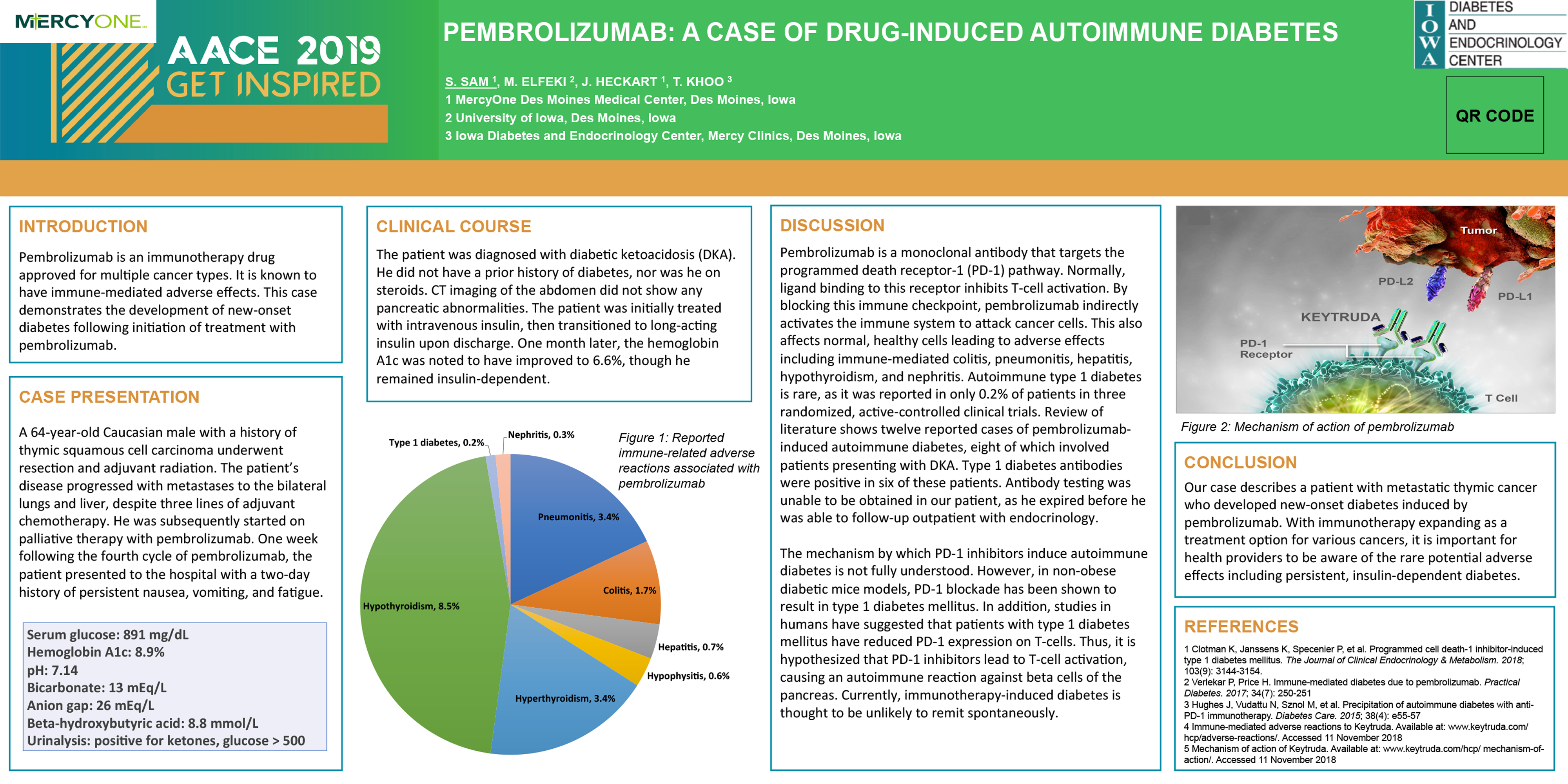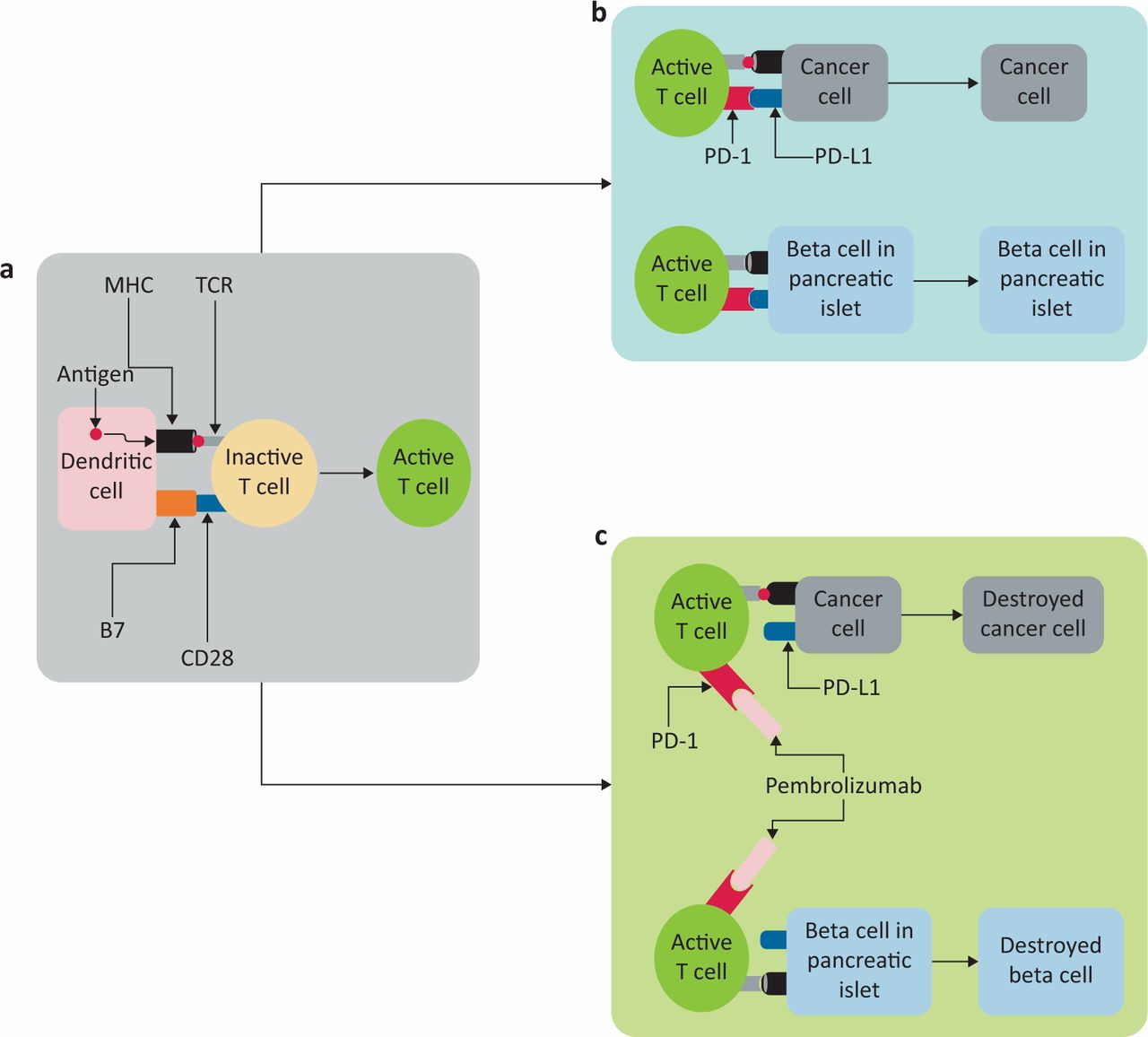
Is immune checkpoint inhibitor-associated diabetes the same as fulminant type 1 diabetes mellitus? | RCP Journals
Merck posts early clinical data on subcutaneous Keytruda, setting stage for further studies | Fierce Pharma

Progression To Insulin Dependence Post-Treatment With Immune Checkpoint Inhibitors In Pre-Existing Type 2 Diabetes - AACE Clinical Case Reports

Moderna und Merck geben bekannt, dass mRNA-4157/V940, ein personalisierter mRNA-Krebsimpfstoff, in Kombination mit KEYTRUDA(R) (Pembrolizumab) primären Endpunkt in Phase-2b-Studie KEYNOTE-942 erreicht hat

Diabetic ketoacidosis following immunotherapy for lung cancer | Tidsskrift for Den norske legeforening


:max_bytes(150000):strip_icc()/VWH_Illustration_Drug_Keytruda-Pembrolizumab_Dennis-Madamba_Final-4a86ef9e12524d42aebbcd4126935dec.jpg)